Want to find out more about any of the above services?
We’d like to hear from you, click below to get in
touch with our experienced sales team!
Predictive Coverage Reports
Before FDA approval, it’s crucial to know how Payers will treat your new drug, device, or test. Understanding coverage, restrictions, and criteria before commercialization allows you to design an effective and efficient market access strategy. Policy Reporter’s Predictive Payer Coverage Report allows you to view critical insight into coverage before your product launch.
Designed for pharmaceutical, medical device, and diagnostics companies who are releasing a new drug, device, or test, Policy Reporter leverages its proprietary historical database of coverage decisions to build a predictive model of likely post-approval coverage scenarios.
What We Deliver
Key Coverage Data:
Which payers are most likely to cover first, second, last
What restrictions and criteria are most likely to be instituted
What tier level is likely to apply to your product
Are there certain HTAs, studies, or guidelines that will be influential on coverage
From FDA Approval to Initial Coverage, a Detailed Analysis
We also include statistics highlighting:
The likelihood of coverage by payer, geography, or plan type
Detailed visual analytics, including graphs, charts, and pivot tables of coverage criteria
A detailed analysis on the Payers most likely to cover first according to average days of FDA approval to initial coverage
An analysis on the Payers most likely to cover last according to average days of FDA approval to initial coverage
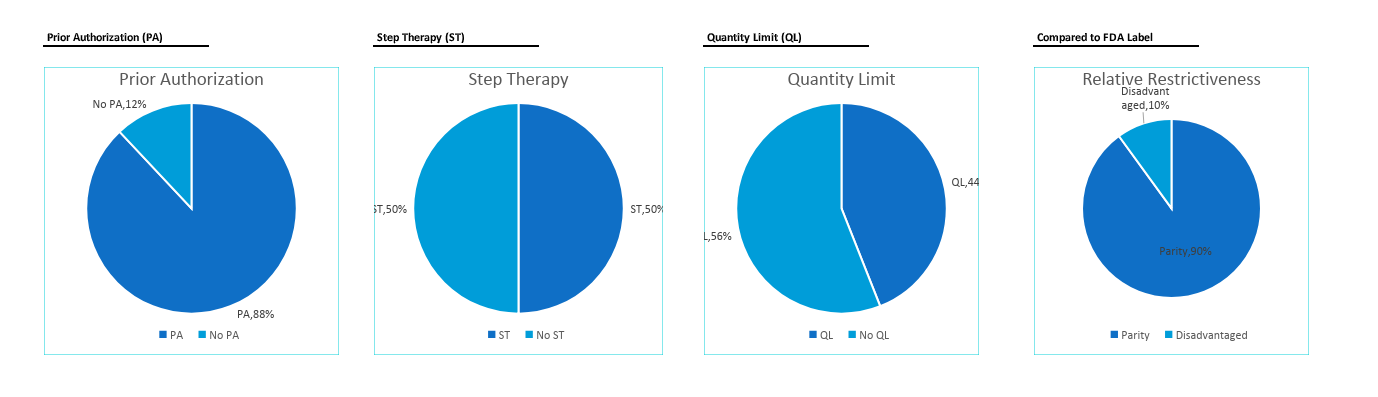
Predictive Coverage Reports offer a comprehensive breakdown of predicted initial coverage and restrictiveness probabilities by Payer.
Pharmaceutical Specific Information
Predictive Coverage Reports can also include:
Formulary tier placement prediction for all payers, including
Restriction and criteria prediction/modeling
Most and least restrictive payers
Analysis of restrictions including testing, indications, diagnostics, frequency limitations, age restrictions, prior authorization, step therapy, and more!
All coverage restrictions on the analog drugs, device, or test for all payers
All formulary restrictions on the analog drugs for all payers
A variety of optional add-ons such as a corresponding sample coverage policy.
Experienced Market Access Strategy Consulting
We expand upon the power of our Predictive Payer Coverage Report by providing comprehensive Market Access, Payer Engagement, and Payer Communication Strategy. Policy Reporter’s extensive experience working with top tier pharmaceutical, medical device, and diagnostic companies allows us to develop a targeted, effective, and efficient strategy for your team.
The strategy begins prior to FDA approval and takes your team through the first 30, 60, and 90 days, and onto the first year, two years, and three years. We identify geographies and accounts to target first, help craft your payer communications, and provide insights into key payer contacts to reach out to. Policy Reporter’s Market Access Strategy Consulting can help your product ensure success during market release and early market adoption.